
17th December 2015 by Jason Vincent
A Guide to CE Marking Products as a Manufacturer – Getting Started
This post is part of a series, and picks up from the introductory post on CE marking which can be seen here.
In the previous post we looked at what CE marking is, and when it is required. We also looked briefly at the different product categories that are covered, together with some of the directives that you may need to consider.
Essential Product Requirements
Before diving in to some of the directives in more detail, let’s have a quick look at what are essential product requirements, and how they are covered by CE marking.
“A fundamental feature of a large part of Union harmonisation legislation is to limit legislative harmonisation to the essential requirements that are of public interest. These requirements deal with the protection of health and safety of users (usually consumers and workers) but may also cover other fundamental requirements (for example protection of property, scarce resources or the environment).
Essential requirements define the results to be attained, or the hazards to be dealt with, but do not specify the technical solutions for doing so. The precise technical solution may be provided by a standard or by other technical specifications at the discretion of the manufacturer. This flexibility allows manufacturers to choose the way to meet the requirements. It allows also that, for instance, the materials and product design may be adapted to technological progress.”
It is the manufacturer’s responsibility to identify the essential requirements of their product. Some of these requirements will be covered by the relevant directives eg: Low Voltage Directive, though some others may not. It is the manufacturer’s responsibility to ensure that identified risks are managed correctly, irrespective of whether or not they fall under one of the applicable directives, and to thoroughly document how the risk is managed.
Overview of Technical Documentation
When it comes to compiling the technical documentation for the product being manufactured, this will most likely be driven by the contents stipulated in the relevant directive. “As a rule, the documentation has to include a description of the product and of its intended use and cover the design, manufacture and operation of the product. […] In the case where a product has been subject to re-designs and re-assessment of the conformity, the technical documentation must reflect all versions of the product; describing the changes made, how the various versions of the product can be identified and information on the various conformity assessment.”
The Technical Documentation must be kept for a minimum period of 10 years (or the minimum length specified by the relevant directive) from the moment the product was first placed on the market.
We will go in to more detail on this later, and provide some examples directly relevant to some of the more common directives.
The Declaration of Conformity
As mentioned before, it is the responsibility of the manufacturer to issue a Declaration of Conformity. “The EU Declaration of Conformity must contain all relevant information to identify the Union harmonisation legislation ac- cording to which it is issued, as well as the manufacturer, the authorised representative, the notified body if applicable, the product, and where appropriate a reference to harmonised standards or other technical specifications.”
Similar to the Technical Documentation, the Declaration of Conformity must be kept for a minimum of 10 years from the moment when the product was first placed on the market.
To save you the time of trying to find an example, you can download a sample declaration of conformity with placeholders from here. This is extracted directly from the Union “DECISION No 768/2008/EC” page 47[3].
As you can see, this document is fairly straightforward, and shouldn’t take too long to compile, provided that the technical documentation has been correctly collated. The information consists of:
- A number identifying the product. This number does not need to be unique to each product. It could refer to a product, batch, type or a serial number. This is left to the discretion of the manufacturer.
- The name and address of the manufacturer or the authorised representative issuing the declaration;
- A statement that the declaration is issued under the sole responsibility of the manufacturer.
- The identification of the product allowing traceability. This is basically any relevant information supplementary to point 1 describing the product and allowing for its traceability. It may where relevant for the identification of the product contain an image, but unless specified as a requirement in the Union harmonisation legislation this is left to the discretion of the manufacturer.
- All relevant Union harmonisation legislation complied with; the referenced standards or other technical specifications (such as national technical standards and specifications) in a precise, complete and clearly defined way; this implies that the version and/or date of the relevant standard is specified.
- The name and identification number of the notified body when it has been involved in the conformity assessment procedure;
- All supplementary information that may be required (for example grade, category), if applicable;
- The date of issue of the declaration; signature and title or an equivalent marking of authorised person; this could be any date after the completion of the conformity assessment.
Applying the CE Mark to your product
The gov.uk site has a basic overview of CE marking that was linked from my first post. Since the requirements for applying the CE mark are fairly straightforward, I have quoted them verbatim below[2].
Depending on the specifics of the directive that covers your product, you must make sure that:
- The initials ‘CE’ are in the standard, recognisable form
- If you reduce or enlarge the size of your marking the letters CE must be in proportion to the standard version
- The CE marking is at least 5 millimetres – unless a larger minimum dimension is specified in the relevant directive
- The CE marking is placed onto the product or to its data plate – if this is not possible or not warranted because of the nature of the product, it must be placed onto the packaging and accompanying documents
- The CE marking is easily visible, readable and permanent
Conformity Assessment by the Manufacturer
So far we’ve looked at what CE marking is, why it is needed, and how the process works at a very high level. Now it’s time to go into a bit more detail about the Conformity Assessment process, as this will provide the basis for compiling your technical documentation. “A product is subjected to conformity assessment both during the design and production phase. Conformity assessment is the responsibility of the manufacturer. Should a manufacturer subcontract design or production, he still remains responsible for the execution of conformity assessment.”
Conformity assessment is the process carried out by the manufacturer of demonstrating whether specified requirements relating to a product have been fulfilled.
Each Directive specifies processes for two important elements:
- The legislative requirements governing the characteristics of the products covered;
- The conformity assessment procedures the manufacturer carries out in order to demonstrate that a product, before it is placed on the market, conforms to these legislative requirements.
The Conformity Assessment process can be carried out in one of three ways, and this is directed by the relevant directives that the product must comply with, and in turn the relevant product category that it falls in to[1].
- In some cases, particularly with low risk products, the conformity assessment can be undertaken by the manufacturer on their own, without any third party involvement. In this case the manufacturer himself carries out all required controls and checks, establishes the technical documentation and ensures the conformity of the production process.
- Conformity assessment is performed with the involvement of an accredited in-house conformity assessment body that forms a part of the manufacturer’s organisation. However this in-house body must not have any activities other than conformity assessment and must be independent from any commercial, design and production entities.
- In some other cases the legislator may consider the intervention of a third party i.e. an external conformity assessment body, necessary. Such a body must be impartial and fully independent from the organisation or the product it assesses, it cannot engage in any activity that may conflict with its independence and thus it cannot have user or other interests in the product to be assessed.
The following flowchart extracted from the source [1] below, illustrates the CE marking process: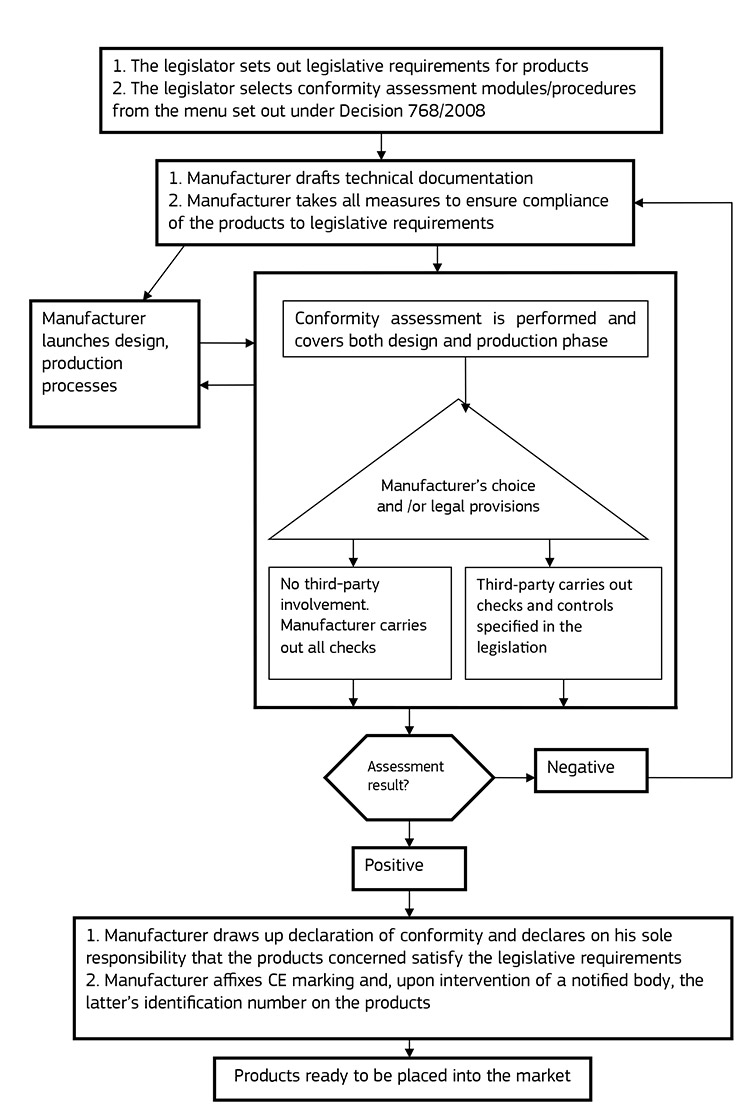
One slightly confusing aspect of conformity assessment is the concept of modules. The conformity assessment process may take place at different stages in the product development and manufacturing process. Which of the modules is relevant to you will be primarily dictated by the relevant directives, and their requirements in order to ensure risks are correctly managed. I have exported pages 59 – 61 of the ‘Blue Guide‘ which you can download here. I recommend you read through this to get a feel for the different modules, and what they cover specifically.
Just to give some examples of how these modules work in practice, module A is simply ‘Internal Product Control’ meaning the manufacturer is undertaking full conformity assessment internally, and this may well be a sole requirement for certain products, covered by the respective directives. However, in some instances you may be required to incorporate module D1 (for instance) which is ‘Quality Assurance of the Production Process’. Which modules are relevant will vary significantly between directives and product categories. I recommend you don’t worry about this excessively for the time being, but it’s useful to at least familiarise yourself with the concept, as it will be referred to going forward. I have included the visual flow-chart representation of the modules, illustrating which ones cover the Design or Manufacturing phases, and which ones span both. Hopefully this will make sense.
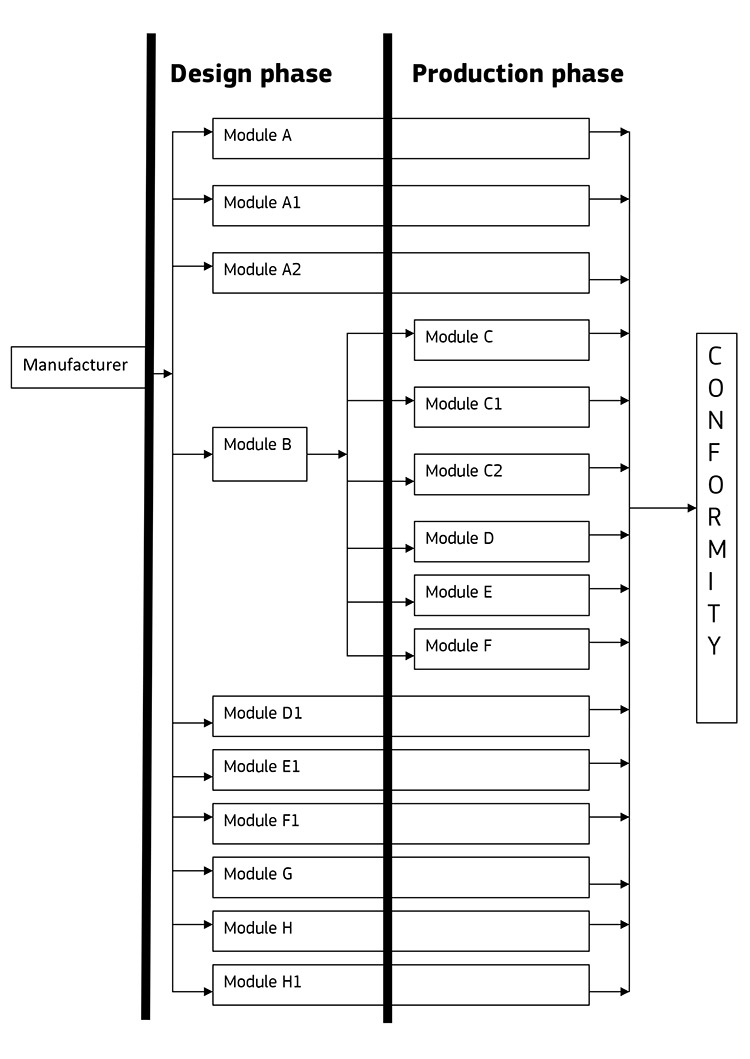
This post should give a good overview of the CE marking process, and more specifically the conformity assessment process. In the next post we’ll take a specific product and look at which directives need to be considered by the manufacturer. We’ll also go into detail on how to compile your technical documentation and carry out the conformity assessment. I hope this has been helpful!
References and additional reading:
[1] http://ec.europa.eu/enterprise/policies/single-market-goods/files/blue-guide/guidepublic_en.pdf
[2] https://www.gov.uk/ce-marking
[3] http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2008:218:0082:0128:en:PDF
[4] http://ec.europa.eu/enterprise/policies/single-market-goods/cemarking/professionals/manufacturers/index_en.htm




